Trioceros hoehnelii
© 2010 Christopher V. Anderson
Our Research
Our research examines the morphological, mechanical and physiological basis of animal movement with an emphasis on how these mechanisms change through evolution and across environments.
General Research Interests:
Research in the Anderson Lab is broadly focused on understanding physiological and biomechanical systems in an ecological and evolutionary context. We examine the morphological, biomechanical and physiological mechanisms underlying animal movement in general, and how these mechanisms change through evolution and vary across environments. We apply an integrative approach, often combining laboratory and field studies, to elucidate interactions between an organism’s physiology and their performance, reveal how organisms perform in their natural surroundings, and improve our understanding of the evolution of form and function. Further, while our research generally utilizes non-model organisms, the mechanistic levels we work at are broadly applicable to a wide range of taxa, including humans, and provide insight into broader functional and physiological principles.
The following are examples of some of the research we are or have been involved in:



Thermal Effects on High-Powered Ballistic Movements
Chameleons, toads and some salamanders feed by way of ballistically projecting their tongue from their mouth to capture prey. These highly specialized feeding mechanisms utilize elastic storage mechanisms, similar in ways to a bow-and-arrow, to launch the tongue. This recoil of elastic elements allows for extreme performance outputs far exceeding that of known muscle properties. My dissertation research focused on the thermal effects of feeding in chameleons (family Chamaeleonidae) in order to understand how explosively dynamic movements powered by elastic recoil are effected by temperature. We discovered that in addition to producing extreme performance, this elastic recoil also liberates tongue projection from much of the normal decrease in performance associated with muscle-powered movements at low temperatures, allowing them to feed at high performance, even at low temperatures where other sympatric lizard species remain inactive. Since this discovery, I have examined this interaction across a range of mechanistic levels and systems. Click here for more information on this project including high-speed videos!
Scaling Patterns of the Chameleon Feeding Mechanism
As organisms grow, their increasing body size results in changes to the way they interact with their environment. An organism’s size and proportions have profound effects on how they are able to move and perform in their surroundings. Similarly, closely related species of different sizes are subject to different constraints on their movements due to their differing body sizes. With collaborators Stephen Deban and Thomas Sheridan, I looked at the scaling patterns of the feeding apparatus of chameleons. I then looked at how these scaling patterns effect tongue projection performance among chameleon species. By looking at how the size and proportions of the different muscles and bones in the chameleon’s feeding apparatus change with body size, and how these changes affect whole organism performance, we are gaining insights into the functional constraints imposed by body size on spring-loaded ballistic movements, as opposed to more typical musculoskeletal systems. Our study examining scaling patterns of the chameleon feeding apparatus was published in the Journal of Morphology and my study looking at scaling effects on tongue projection performance was published in Scientific Reports.
Functional Perspectives on Patterns of adaptive radiation in Anolis lizards
Island anoles are a classic example of adaptive radiation and convergent evolution, with distinct ecotypes of consistent morphological and performance characteristics evolving repeatedly to particular structural habitats on each island. In mainland anoles, on the other hand, the morphology-habitat use relationship varies from that of the island species. In collaboration with Dr. Thomas Roberts we are exploring the differences in the adaptive radiations of mainland and island Anolis lizards. By examining the morphology, performance, and muscle physiology of locomotor and feeding systems in island and mainland anole species originating from different habitat types, we are working to determine if island and mainland anoles have reached functional convergence without phenotypic convergence. In the process, we are also probing the mechanical link between organismal and muscular performance to determine the contractile characteristics that limit organismal performance on different substrate orientations.
More to Come!
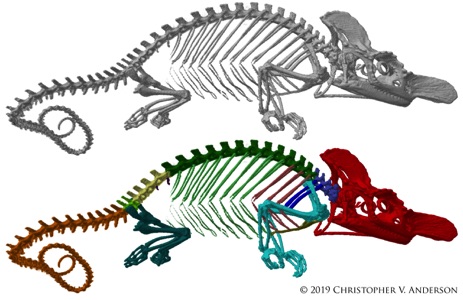
Scanning All The Chameleons! Insights into the Functional & Evolutionary Morphology of Chameleons from Micro-CT Scans
chameleons are well known for their numerous highly characteristic anatomical and behavioral features, such as their projectile tongue, prehensile tail, independently rotating eyes, and color changing abilities. With over 200 described taxa in twelve genera, however, chameleons are extremely diverse in their own right and come in a great variety of unique shapes and sizes. Many of these anatomical variations are poorly understood in an evolutionary, ecological and functional context. We are working to obtain microcomputed tomography (micro-CT) scans of as many chameleon species as possible to examine various anatomical features to provide insights into the evolution and function of skeletal elements in chameleons across disparate ecological environments. So far we have made micro-CT scans of 233 specimens, representing 155 species (and 5 additional subspecies), including all 12 genera of chameleons! That is nearly 75% of all described chameleon species!